Customer Question – What’s this pH all about?
We received the following query through our website:
“Hi, I just wanted to know why soil A & B requires a higher pH level then hydro and cocos? What happens in soil substrate that makes it different? I love science, can you plz explain?
Bill”
We thought our response might help others out there with similar questions.
Hi Bill,
Thanks for getting in touch,
That’s a fairly complex question, with no simple answer. But I’ll try to give you a basic run down of my understanding, and some links for further reading.
Firstly, I take it you understand the pH is a level of acidity/alkalinity of an aqueous or soil substrate. It essentially measures the concentration of hydrogen ions in a solution.
The pH of a substrate influences the ‘cation exchange capacity’ of a soil, that is, it’s ability to hold and exchange different ions. Ions are positively or negatively charged compounds (divided into cations or anions, e.g Ammonium NH4+ or Nitrate N03- ) that interact to feed the plant, bacteria and fungal life in the soil.
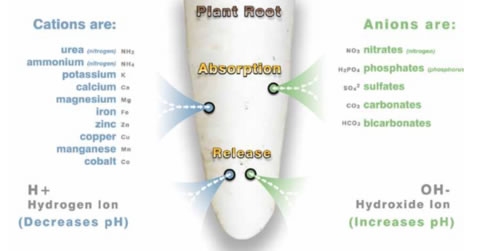
The ability for any plant to uptake nutrients is influenced by this exchange of compounds in the soil and in hydroponic solutions, as shown in the pH availability chart (attached). The different positive and negative compounds, bacteria, water, and other various lifeforms, enzymes and bits and pieces all interact in this delicious soil melting pot to change forms to ideally benefit roots and microbial activity.
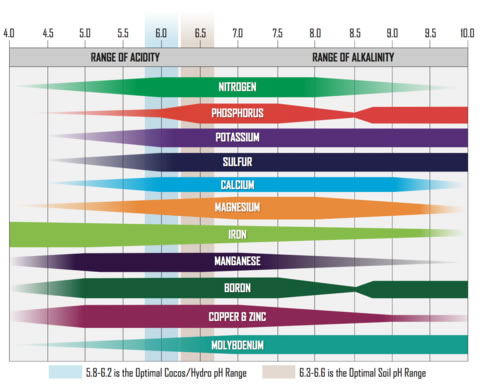
Hydroponic solutions are calculated so that the A/B nutrients and additives all mix harmoniously to achieve a stable pH with the right compounds for a plant to easily absorb.
In soil, the natural bacteria and enzymes play a more significant role in breaking down the different compounds into absorbable forms. It’s also shown that various forms of beneficial bacteria/mycorrhizae and trichoderma populate better in slightly less acidic conditions (e.g. closer to pH 6.5, but remember that pH is a logarithmic measure).
We also have to take into account that different pH levels can change the likelihood of various root diseases and negative organisms occurring in a substrate or water solution. Commercial hydroponic facilities generally run a fairly acidic solution (5.5 – 5.8) as this partially decreases the chance of root disease such as pythium. But the aeration, moisture content, water temperatures, dissolved oxygen, water movement and other factors also all contribute to bacterial populations and interactions, positive and negative.
With effective plant nutrition in soil or hydroponics, a combination approach of mineral nutrients (in easily absorbable compounds), chelating acids (humid & fulvic) and organic additives (sea kelps etc that must break down to feed the plant/beneficial bacteria), all work symbiotically to provide the plant with a full and healthy diet, ensuring it can reach it’s full phenotypic potential.
So it’s not quite as simple as ’the plant gets more Nitrogen at pH 6.0 than 7.0’ as theres a plethora of factors all contributing to the different compounds and living organisms at work. But hopefully this gives you an idea why pH is crucially important to crop nutriment, and why there are so many theories about the ‘best’ approach of feeding your favourite plants!
References and Reading material:
Make sure to download our H&G Growers Journal for free at this link:
https://stealth-garden.com/pages/resources
It has a lot of useful information about various products, how they work and how to best cultivate healthy crops!
Also I’ve done a quick look around and found a couple interesting bits of relevant reading:
http://www1.agric.gov.ab.ca/%24department/deptdocs.nsf/all/agdex6607
http://extension.wsu.edu/publications/wp-content/uploads/sites/54/publications/fs195e.pdf
https://www.ctahr.hawaii.edu/oc/freepubs/pdf/PD-106.pdf
If you want the ultimate botany reference, this is my preference:
You can buy it online, or read small exerts from each chapter on their website.
Hope that helps mate,
Cheers,
Tom
Victorian Sales, Technical Support & Communications
